Developing CRAC
Channel Inhibitors for
Acute Inflammatory
Diseases of High
Unmet Need
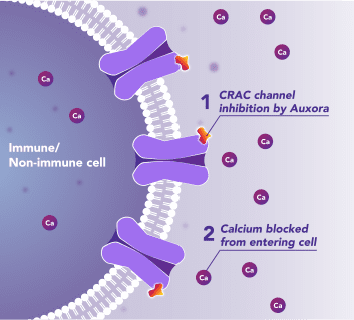
Auxora™’s Dual Mode of Action: As a CRAC Channel Inhibitor, Auxora™ Modulates the Immune Response and Protects Against Tissue Cell Injury
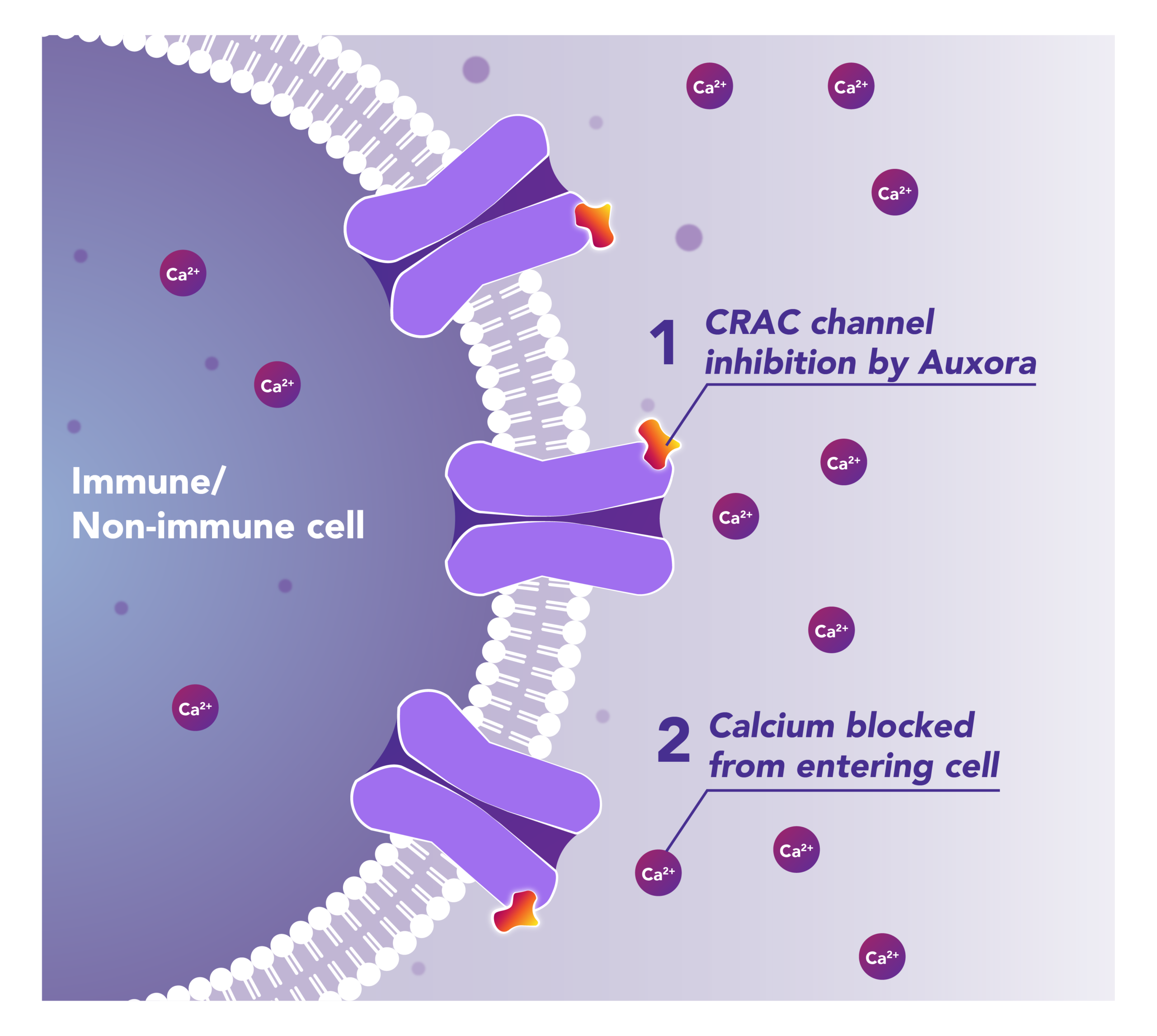
Preclinical and clinical data have demonstrated that the inhibition of disease-activated CRAC channels may have a therapeutic effect based on a dual mechanism involving both anti-inflammatory and tissue cell protective activities.
CalciMedica is developing Auxora™ as a potential disease-modifying therapy for patients with acute life-threatening inflammatory diseases, including AKI and AP, for which there are currently no approved therapies.
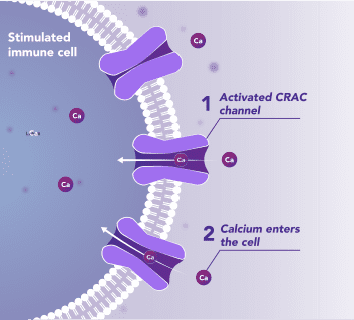
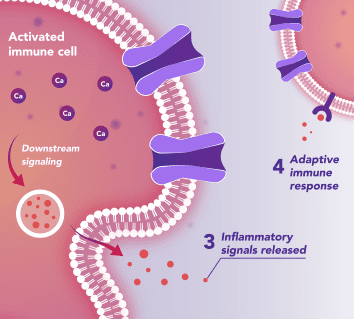
Immune Cells:
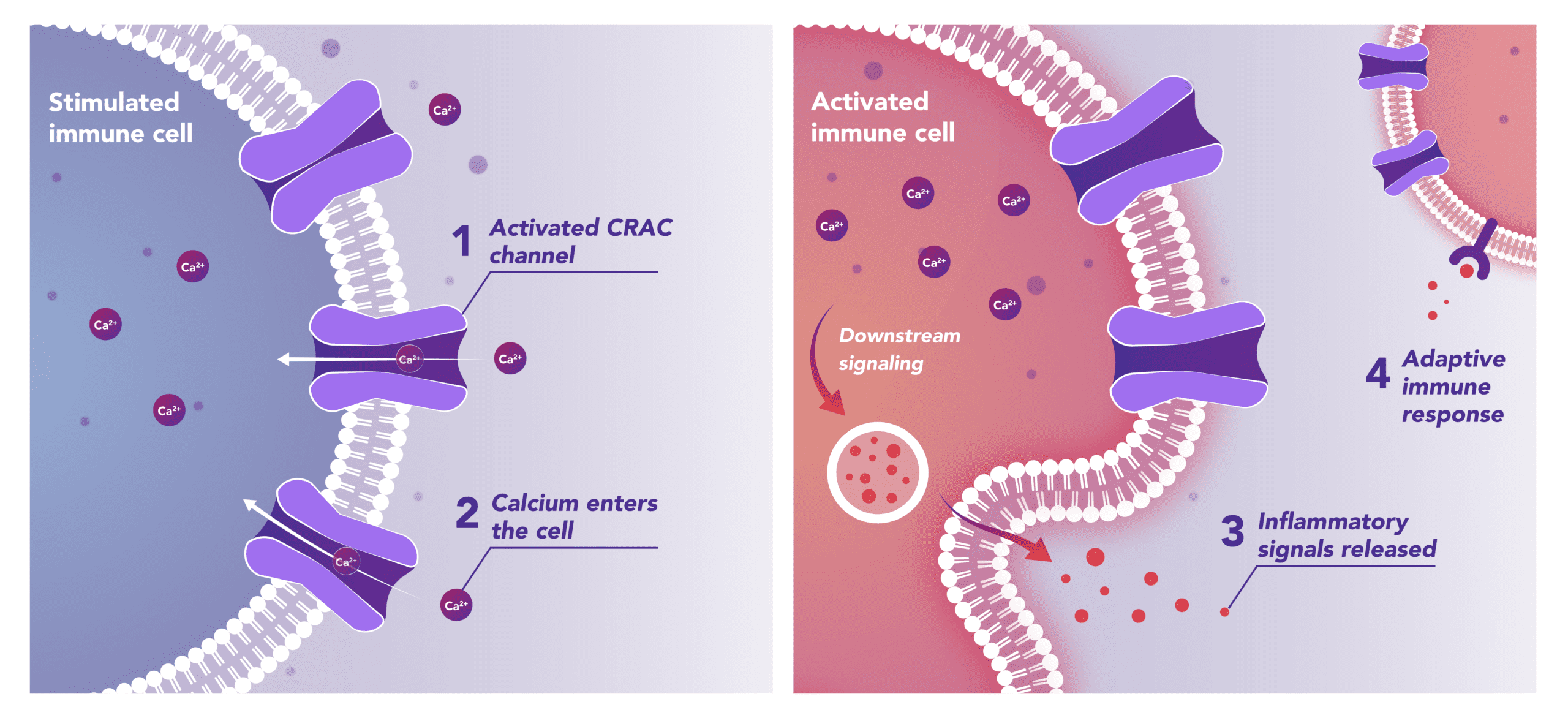
Modulates Immune Response
In immune cells, activation of CRAC channels in response to disease or trauma is a key step in initiating the adaptive immune response and the generation of inflammatory signals such as cytokines. CRAC channels are also important in mediating immune cells that drive the innate immune response. Inhibiting CRAC channels with Auxora™ may reduce immune cell activation and the accompanying inflammation driven by the immune response.
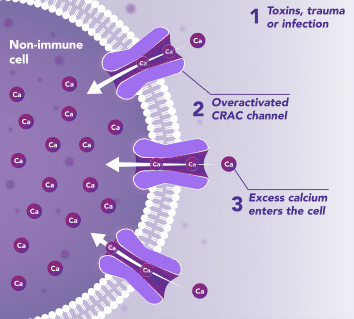
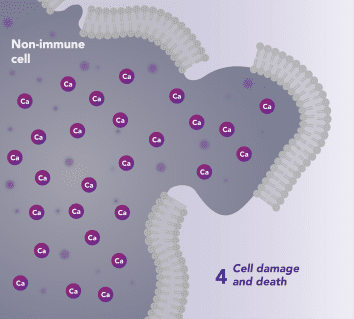
Non-Immune Cells:
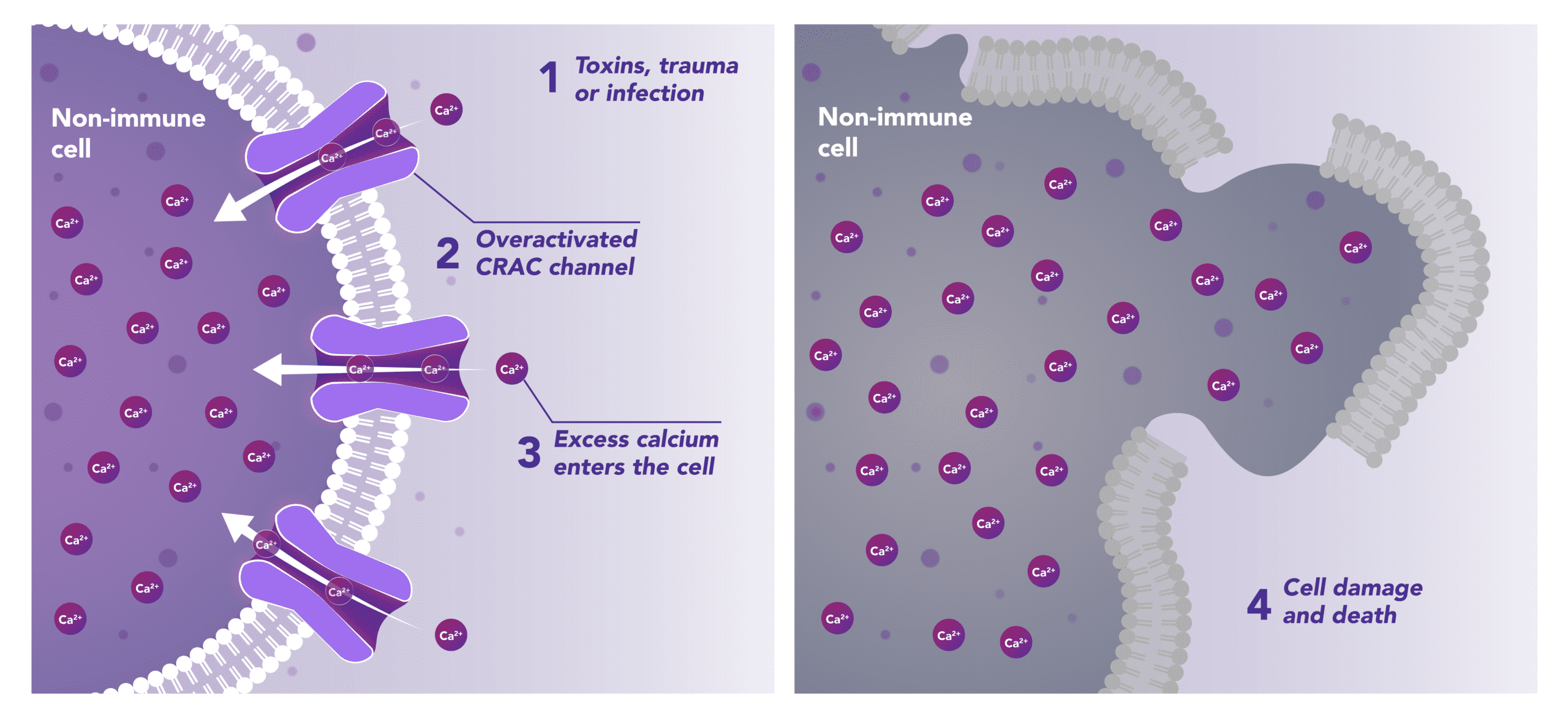
Blocks Calcium from Entering the Cells
CRAC channels on affected organ tissue cells can become inappropriately activated or overactivated, resulting in excess calcium entry into cells. This excess calcium can cause cellular injury and necrosis or activate apoptosis signaling pathways leading to programmed cell death, which is further exacerbated by the damage caused by the inflammatory response. Inhibiting CRAC channels with Auxora™ may reduce organ damage by blocking excess calcium.
“Since the founding of CalciMedica, we have advanced research on CRAC channel inhibitors and their role in acute and chronic inflammatory illnesses to become a leader in the clinical application of this technology. Auxora, our proprietary intravenous-formulated CRAC channel inhibitor, has shown significant potential to treat life-threatening acute inflammatory diseases by modulating the immune response and reducing organ cell damage.”

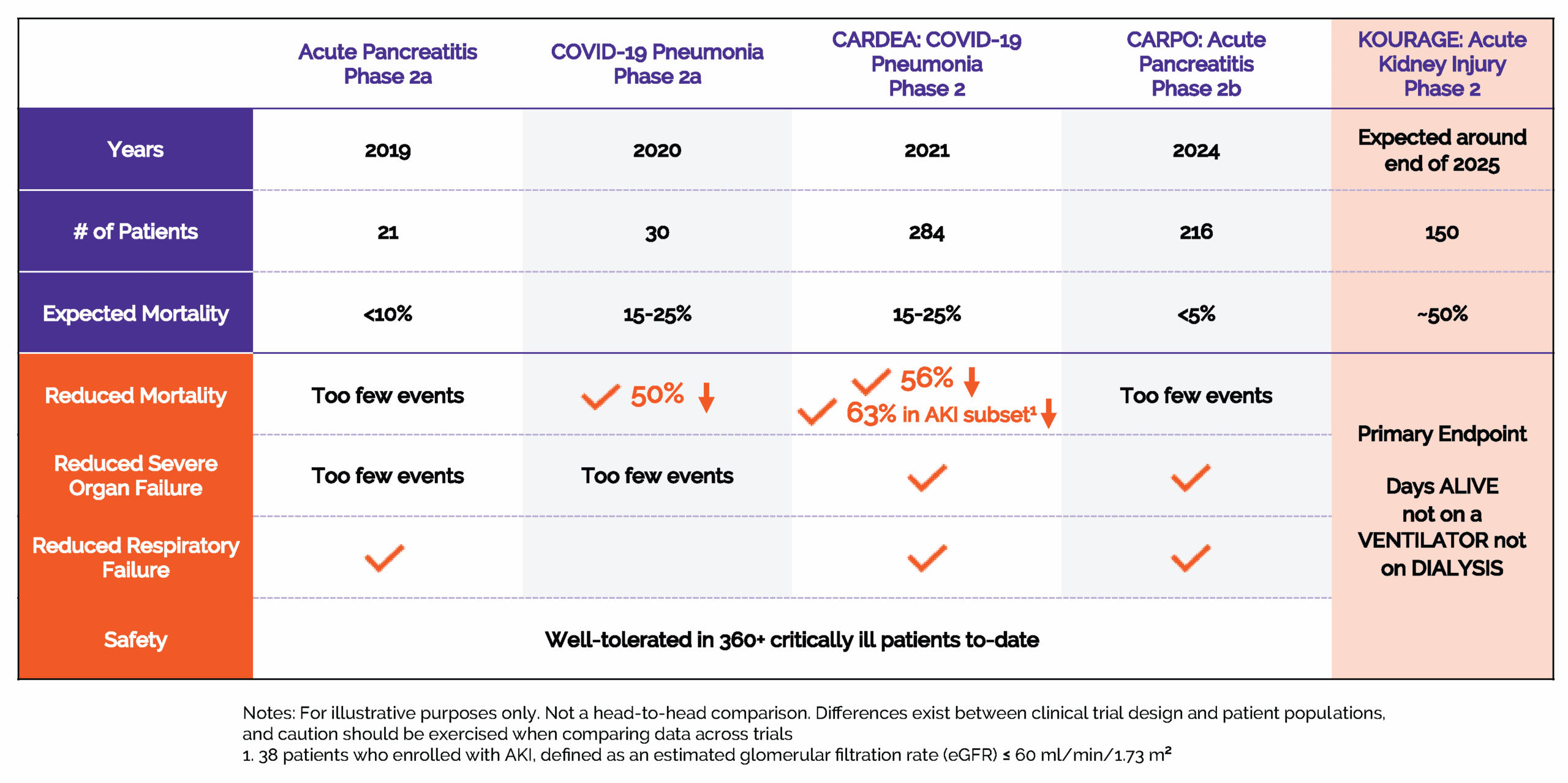
CalciMedica’s lead pipeline candidate, Auxora™, is a proprietary, intravenous-formulated, small molecule CRAC channel inhibitor. Auxora™ has been studied in four Phase 2 clinical trials, demonstrating positive and consistent clinical results as well as a favorable safety profile, and is currently being studied in two additional Phase 2 clinical studies.
| Program1 | Indication | Phase of Development | Anticipated Milestones in next 12 months | |||
| Preclinical | Phase 1 | Phase 2 | Phase 3 | |||
| Acute Disease (IV) | ||||||
| Auxora | Acute Pancreatitis | Final pivotal trial design 1H 2026 | ||||
| Auxora | Acute Kidney Injury with Respiratory Failure |
Analysis of discontinued KOURAGE trial |
||||
| Auxora | Asparaginase-Induced Pancreatic Toxicity in Pediatric Patients | Update – 2026 | ||||
| Chronic Disease (Oral) | ||||||
| CM5480 | Pulmonary Arterial Hypertension | Add’l preclinical data – 2H 2026 Initiate Ph1 – 2027 |
||||
| Rheumatoid Arthritis | Lead Optimization | |||||
with Respiratory Failure
KOURAGE trial
Initiate Ph1 – 2027
AP hospitalizations per year in the US, with 170K having predicted severe disease
- Standard of care: Supportive care – IV fluids, pain medication, and nutrition
- Severe complications include pancreatic necrosis, life-threatening distal organ failure, and acute respiratory distress syndrome (ARDS)
- A major economic burden: >1M+ patient days in hospital per year and >$3B cost per year
Stage 2 or 3 AKI patients in the ICU each year, 800K of whom have concurrent respiratory failure
- Standard of Care: Supportive care Treatment of complications, dialysis
- Disease can progress to:
- Chronic kidney disease
- End stage renal disease
- Eventual Death
- 90-Day Mortality: ~50%
CalciMedica’s Proprietary Technology Targeting CRAC Channel Inhibition for the Treatment of Inflammatory Diseases of High Unmet Need
“Acute Kidney Injury with Respiratory Failure and Acute Pancreatitis are two life-threatening acute inflammatory illnesses for which doctors have very little to offer aside from supportive care. In our Phase 2 clinical trials, we have consistently demonstrated that patients treated with our lead compound, Auxora™, benefited from reduced mortality, reduced severe organ failure, improved outcomes with earlier discharge from the hospital, and fewer follow-on complications. We are encouraged by these findings as we continue our quest to improve the lives of patients with acute inflammatory illnesses.”

We have studied Auxora™ in multiple clinical trials including four Phase 2 clinical trials.
We believe the consistency of the clinical results we observed from these four trials in multiple acute critical care conditions affecting different primary organs are mutually supportive and reinforce our plans to develop Auxora in acute critical illnesses such as acute kidney injury (AKI) and acute pancreatitis (AP).