Targeting CRAC
Channel Inhibition for
Inflammatory Diseases
of High Unmet Need
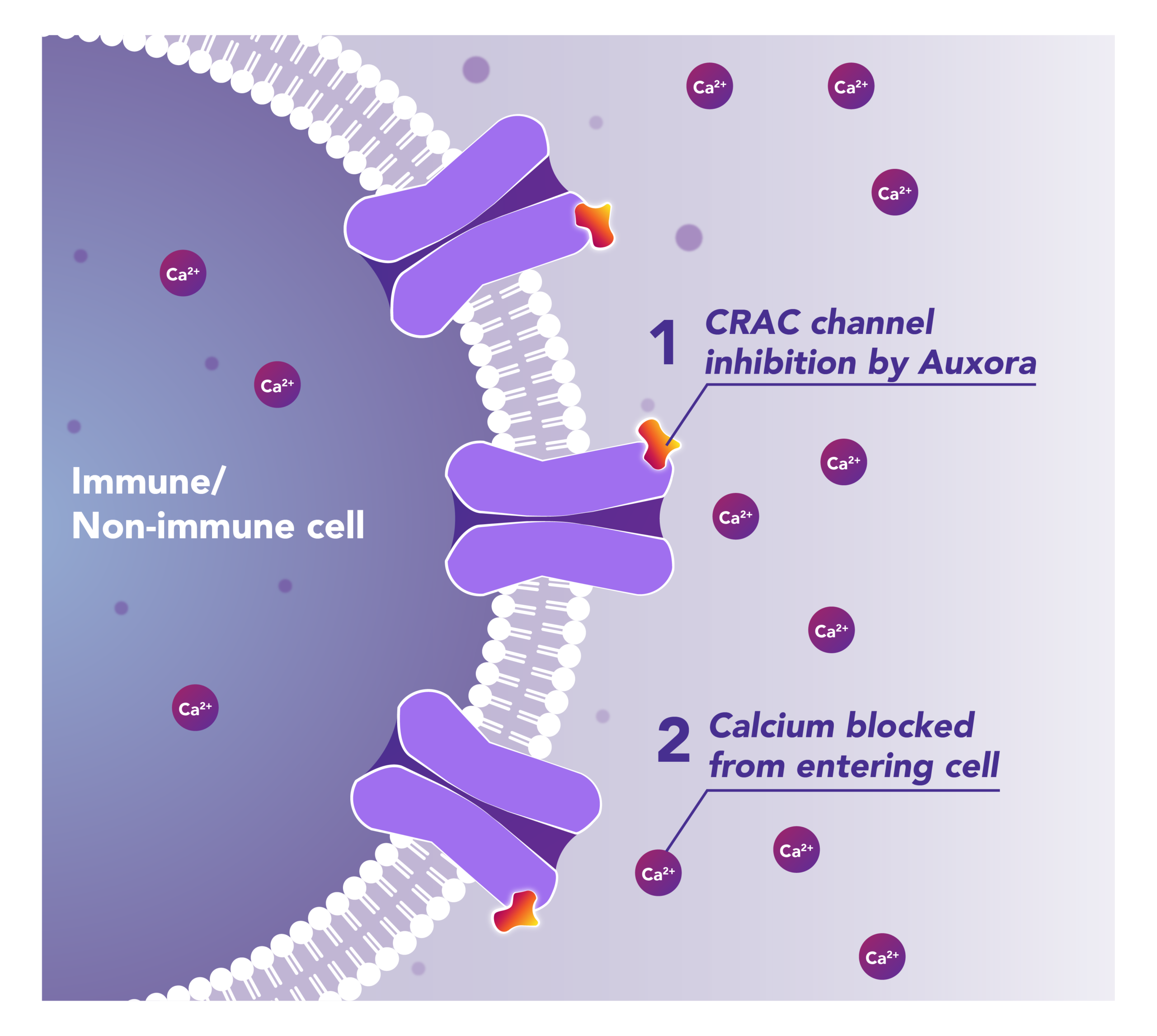
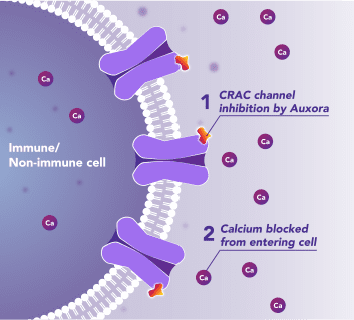
Auxora™’s Dual Mode of Action: As a CRAC Channel Inhibitor, Auxora™ Modulates the Immune Response and Protects Against Tissue Cell Injury
Preclinical and clinical data have demonstrated that the inhibition of CRAC channels may have a therapeutic effect based on a dual mechanism involving both anti-inflammatory and tissue cell protective activities.
CalciMedica is developing Auxora™ as a potential disease-modifying therapy for patients with life-threatening inflammatory diseases of the pancreas, kidney and lung, for which there are currently no approved therapies.
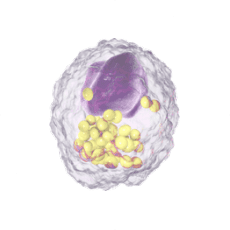
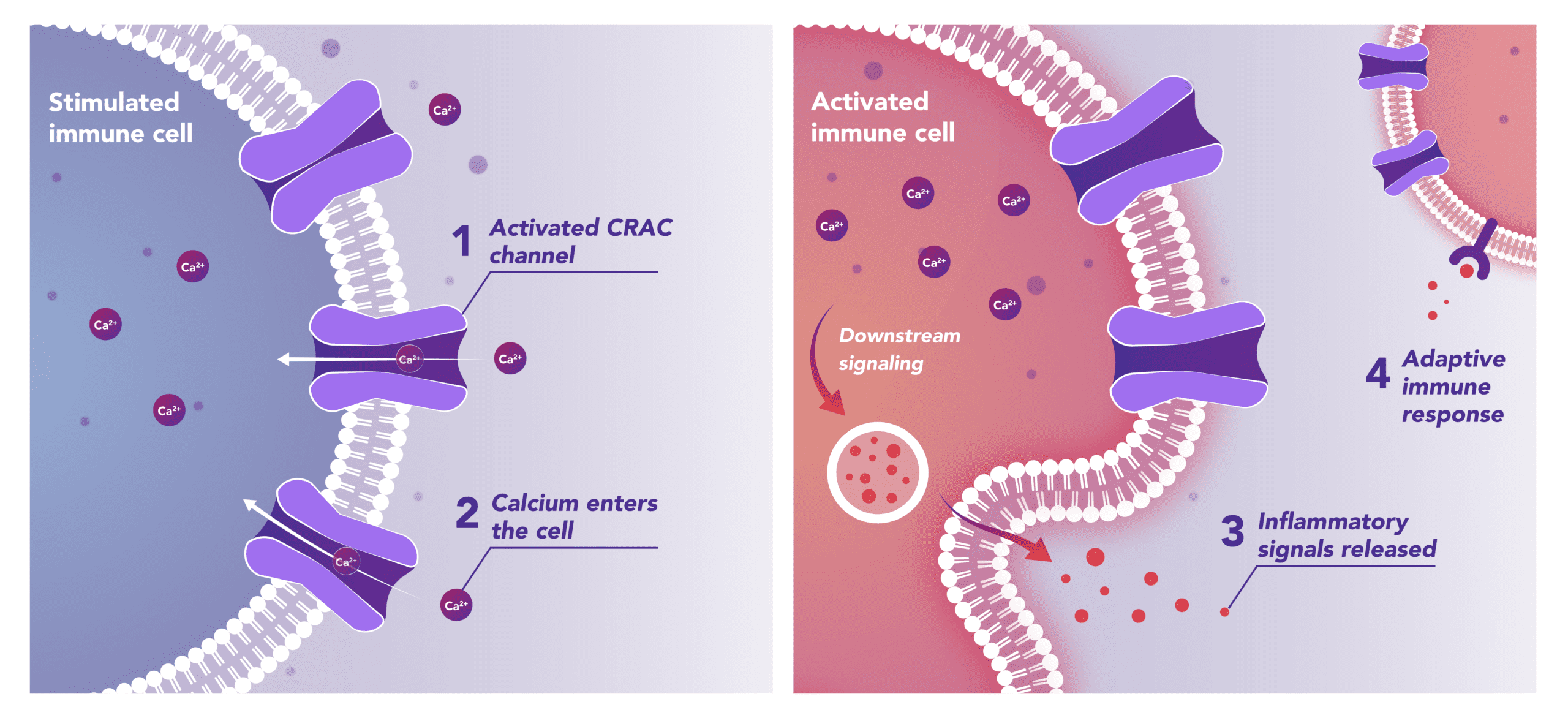
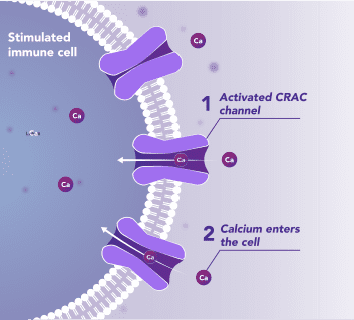
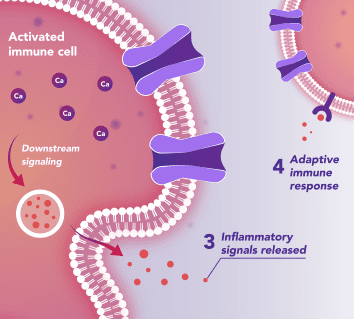
Immune Cells:
Modulates Immune Response
In immune cells, activation of CRAC channels is a key step in initiating the adaptive immune response and the generation of inflammatory signals such as cytokines. Inhibiting CRAC channels with Auxora™ may reduce immune cell activation and the accompanying inflammation driven by the immune response.
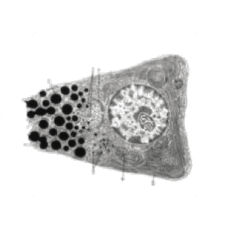
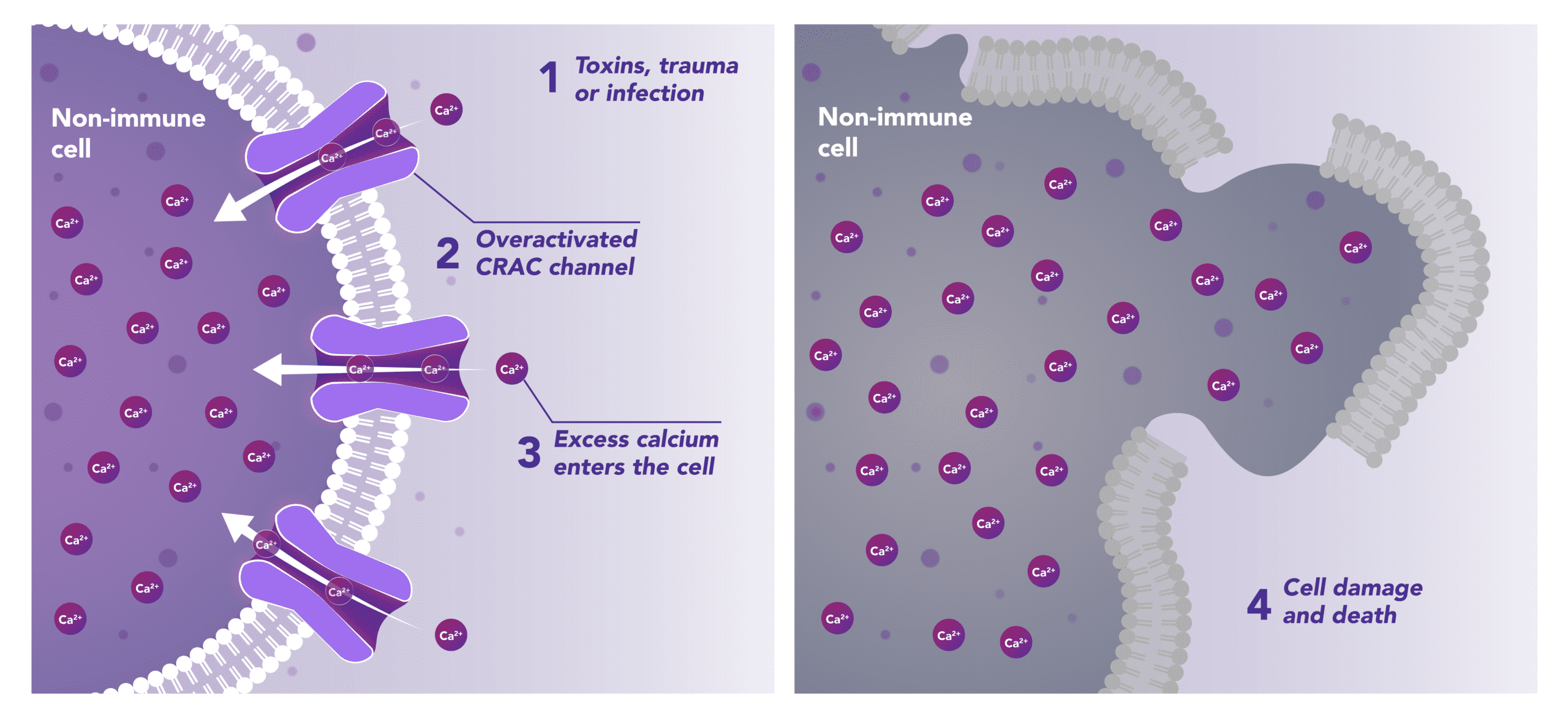
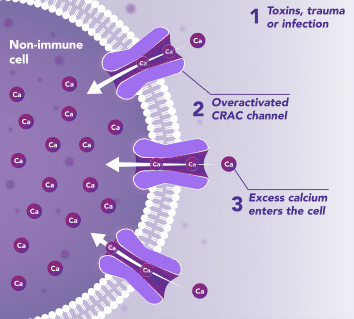
Non-Immune Cells:
Blocks Calcium from Entering the Cells
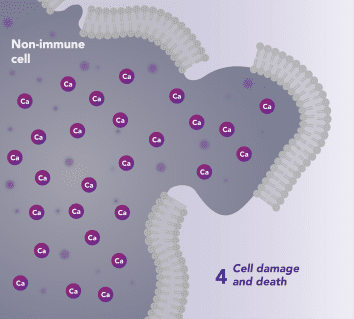
CRAC channels on affected organ tissue cells can become inappropriately activated or overactivated, resulting in excess calcium entry into cells. This excess calcium can cause cellular injury and necrosis or activate apoptosis signaling pathways leading to programmed cell death, which is further exacerbated by the damage caused by the inflammatory response. Inhibiting CRAC channels with Auxora™ may reduce organ damage by blocking excess calcium.
“Since the inception of CalciMedica more than 15 years ago, we have advanced our research of CRAC channel inhibitors and their role in acute and chronic inflammatory illnesses. We believe the potential for Auxora, our proprietary intravenous-formulated CRAC channel inhibitor, to treat life-threatening inflammatory diseases by modulating the immune response and reducing organ cell damage, is significant.”

CalciMedica’s lead pipeline candidate, Auxora™, is a proprietary, intravenous-formulated, small molecule CRAC channel inhibitor. Auxora™ has been studied in four Phase 2 clinical trials, demonstrating positive and consistent clinical results as well as a favorable safety profile, and is currently being studied in two additional Phase 2 clinical studies.
| Program1 | Indication | Phase of Development | Anticipated Milestones | |||
| Preclinical | Phase 1 | Phase 2 | Phase 3 | |||
| Acute Disease (IV) | ||||||
| Auxora | Acute Pancreatitis | End-of-Phase 2 Meeting with FDA | ||||
| Auxora | Acute Kidney Injury with Respiratory Failure |
KOURAGE Phase 2 trial enrolling; data expected around end of 2025 | ||||
| Auxora | Asparaginase-Induced Pancreatic Toxicity in Pediatric Patients | Update in 2H 2025 | ||||
| Chronic Disease (Oral) | ||||||
| Chronic Pancreatitis | Lead Optimization | |||||
| Rheumatoid Arthritis | Lead Optimization | |||||
with Respiratory Failure
Hospitalizations per year in the US, with one third having or developing SIRS, indicative of predicted or severe disease
- Standard of care: Supportive care – IV fluids, pain medication, and nutrition
- Severe complications include pancreatic necrosis, life-threatening distal organ failure, and acute respiratory distress syndrome (ARDS)
- 20-30% mortality rate in patients with severe disease
Children with acute lymphoblastic leukemia (ALL) treated with chemotherapeutic asparaginase in US annually, 7-10% of whom develop AIPT
- Standard of care: Supportive care – IV fluids, pain medication, and nutrition
- >50% of pediatric AIPT patients develop pancreatic necrosis and/or pancreatic pseudocysts
- AIPT requires that asparaginase treatment be halted beyond this there can be other short- or long-term consequences
30% of 3.7MM patients hospitalized annually in the US with AKI are Stage 2 and Stage 3
- Standard of Care: Supportive care Treatment of complications; dialysis
- Disease can progress to:
- Chronic kidney disease
- End stage renal disease
- Eventual Death
Patients per year in the U.S.
3M Patients per year globally
- Standard of care: Oxygen and fluid management; invasive mechanical ventilation
- 10% of intensive care unit admissions worldwide each year. 75-80% moderate or severe. Life-threatening complications including organ damage or organ failure
- 30-40% mortality rate. Leads to 75,000 deaths annually in the U.S
CalciMedica’s Proprietary Technology Targeting CRAC Channel Inhibition for the Treatment of Inflammatory Diseases of High Unmet Need
“In the U.S. alone, nearly 300,000 people are admitted to the Emergency Room each year suffering from acute pancreatitis, one of several life-threatening inflammatory illnesses for which doctors have very little to offer aside from supportive care. In our Phase 2 clinical trials, we demonstrated that patients treated with our lead compound, Auxora™, benefited from improved outcomes, including the ability to tolerate solid food sooner, with earlier discharge from the hospital and fewer follow-on complications. We are encouraged by these findings as we continue our quest to improve the lives of patients with acute inflammatory illnesses.”