
Acute Respiratory Distress
Syndrome (ARDS)
Patients suffering from moderate and severe ARDS as a result of COVID-19 infection who were treated with Auxora™ in addition to standard of care recovered faster than those on standard of care alone, with a 67.8% lower incidence of invasive mechanical ventilation or death during the study period, which was statistically significant (P<0.05).
Decrease in mortality
at Day 30
Reduction in the need for mechanical ventilation
Reduction in reported acute
kidney injury
Day shorter hospital stay
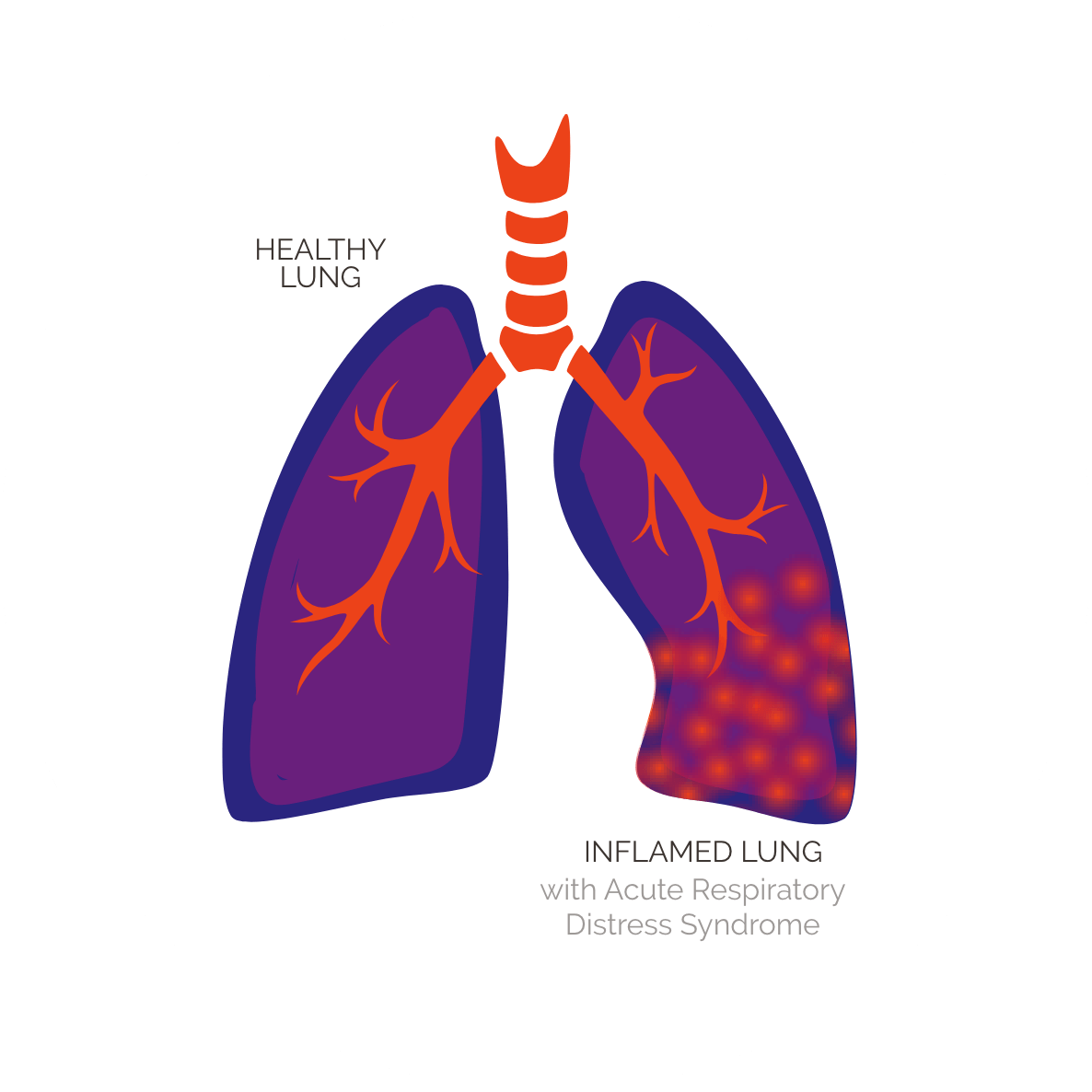

Acute Respiratory Distress
Syndrome
Acute respiratory distress syndrome (ARDS) is a condition in
which fluid builds up in the lungs’ air sacs, or alveoli, limiting
air flow and oxygenation of the blood and thus depriving
organs of oxygen. It typically occurs in patients who are
critically ill or who have significant injuries. It is fatal in
30-40% of patients, and leads to 75,000 deaths annually in the U.S.
The standard of care for patients with ARDS is supportive
care, such as supplemental oxygen and fluid management.
Invasive mechanical ventilation is sometimes required for
these patients.
There are no approved therapeutics for ARDS.



Auxora™: Solving the Unmet
Need For Patients With Acute
Respiratory Distress Syndrome
patients per year in the U.S.
- Organ damage
- Organ failure
- Blood clots
- Infections


Auxora™: Solving the Unmet
Need For Patients With Acute
Respiratory Distress Syndrome
We completed a Phase 2 clinical trial of Auxora™ (CARDEA) in hospitalized, severe and critical COVID-19 pneumonia patients on oxygen.
In this study, we found that in patients treated with Auxora™ plus standard of care, overall mortality was significantly reduced. In addition, these patients demonstrated a trend toward a faster median time to recovery than those treated with standard of care plus placebo.
We completed a Phase 2 clinical trial of Auxora™ (CARDEA) in hospitalized, severe and critical COVID-19 pneumonia patients on oxygen.
In this study, we found that in patients treated with Auxora™ plus standard of care, overall mortality was significantly reduced. In addition, these patients demonstrated a trend toward a faster median time to recovery than those treated with standard of care plus placebo.
Clinical Trials
CARDEA Phase 2 clinical trial in COVID-19 pneumonia patients on ventilators
Study Design
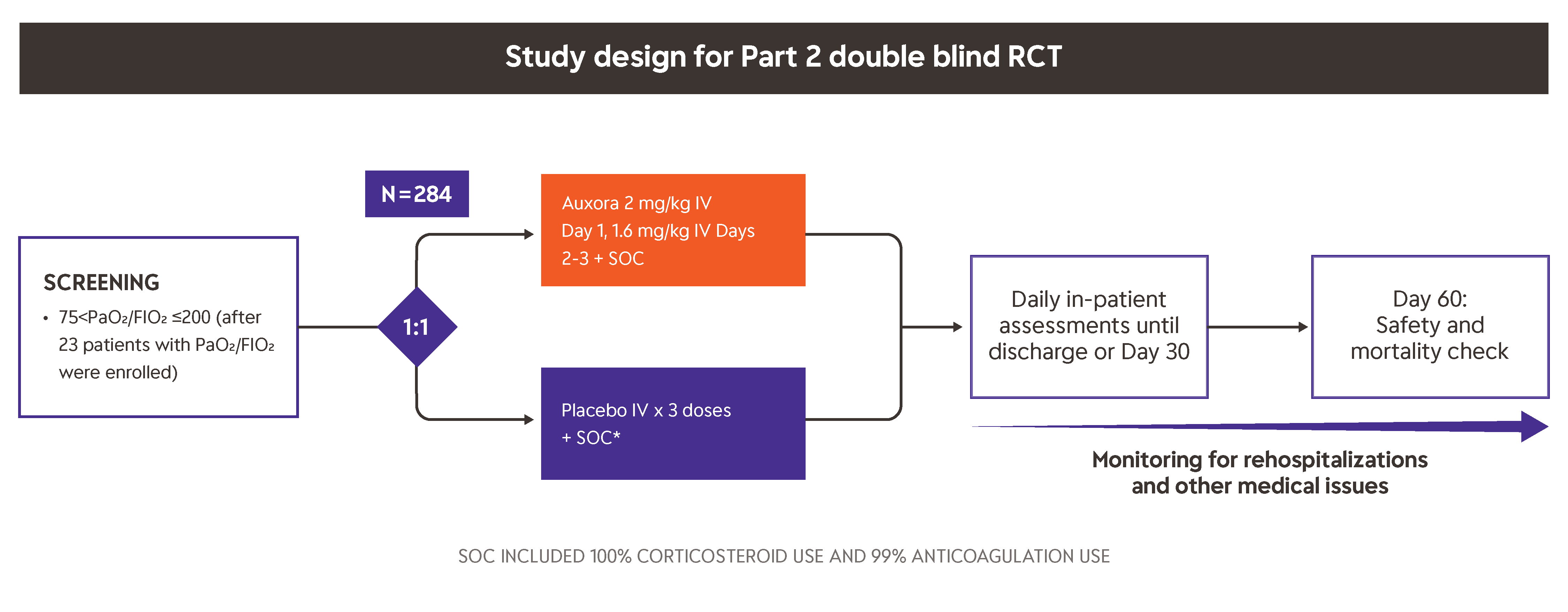
This trial was initially conducted as a Phase 2 randomized, open-label clinical trial in severe COVID-19 pneumonia patients with varying degrees of respiratory failure where time to recovery, blood oxygen levels, ventilator use, and mortality were evaluated. The first safety analysis of the data suggested that patients receiving Auxora™ in addition to standard of care (SOC) were less likely to be placed on mechanical ventilation or to die, and they experienced a reduced time to recovery than those treated with SOC alone.
After 30 patients were enrolled in the first part of the trial (Part 1), the U.S. Food and Drug Administration recommended that Auxora™ be studied in a blinded, placebo-controlled trial.
Part 2 of this trial was initiated in September 2020, and 284 severe and critical but not mechanically-ventilated COVID-19 pneumonia patients were enrolled in this randomized, double-blind, placebo-controlled clinical trial. Patients treated with Auxora™ experienced a reduced time to recovery, higher rate of recovery and reduced mortality.
Results
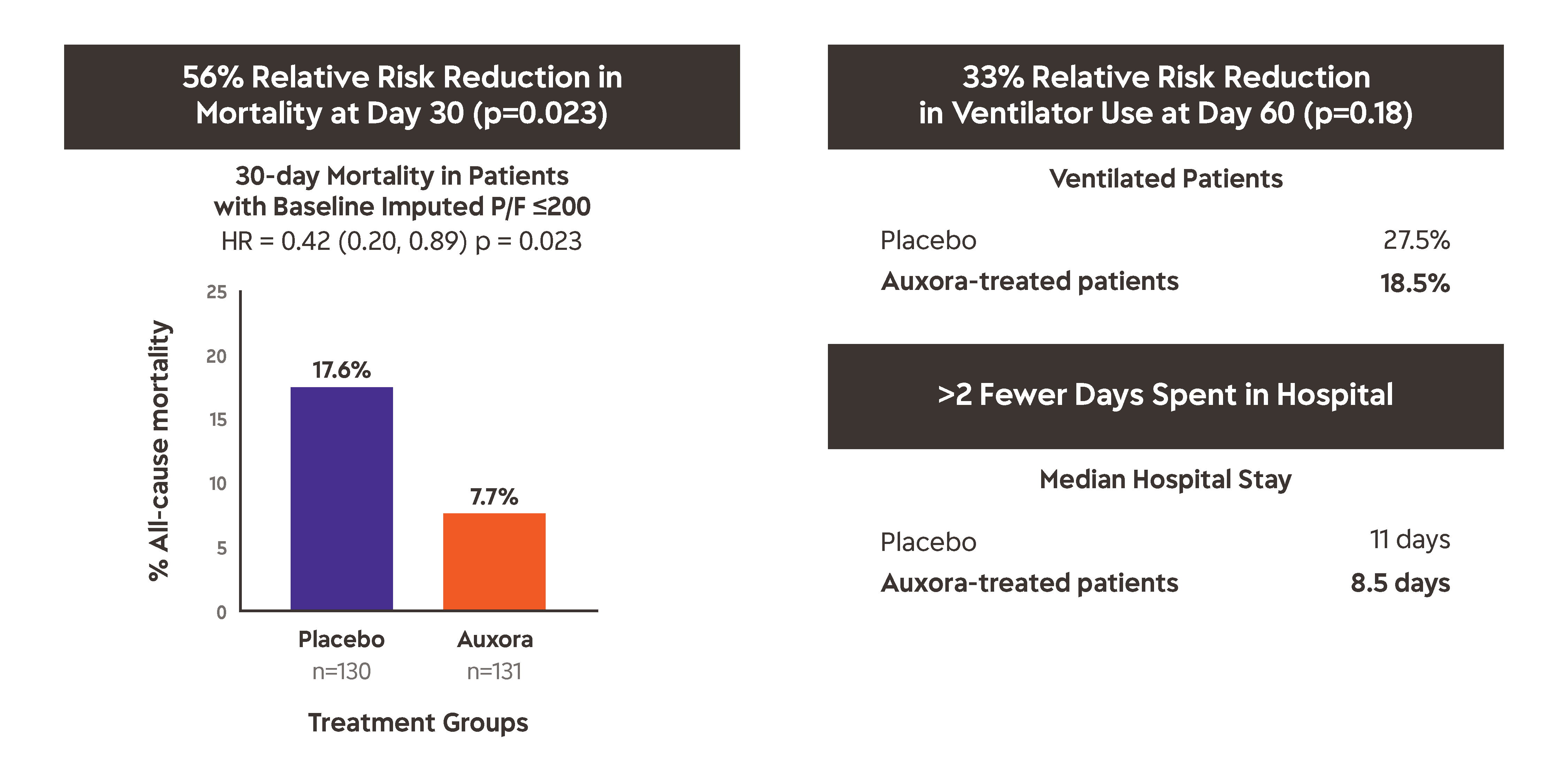
The major findings of the CARDEA Phase 2 trial, as shown above and as follows:
- Time to recovery was seven vs. ten days (P = 0.0979) for patients who received Auxora™ vs. placebo, respectively.
- Day 30 all-cause mortality was 7.7% with Auxora™ vs. 17.6%, with placebo (P = 0.0165).
- Day 60 all-cause mortality was 13.8% with Auxora™ vs. 20.6% with placebo (P = 0.1449).
- Serious adverse events occurred in 24.1% of patients treated with Auxora™ vs. 35% of patients receiving Placebo (P=0.0616)
For more details, please visit A Study of Auxora in Patients With Severe COVID-19 Pneumonia and the Critical Care papers published in 2020 and 2022.

