
Dedicated To Developing
Novel Therapies for
Life-Threatening Acute
Inflammatory and
Immunologic Diseases
CalciMedica is a clinical-stage biopharmaceutical company focused on developing novel CRAC (calcium release-activated calcium) channel inhibition therapies for acute inflammatory and immunologic diseases, including acute kidney injury (AKI) and acute pancreatitis (AP).
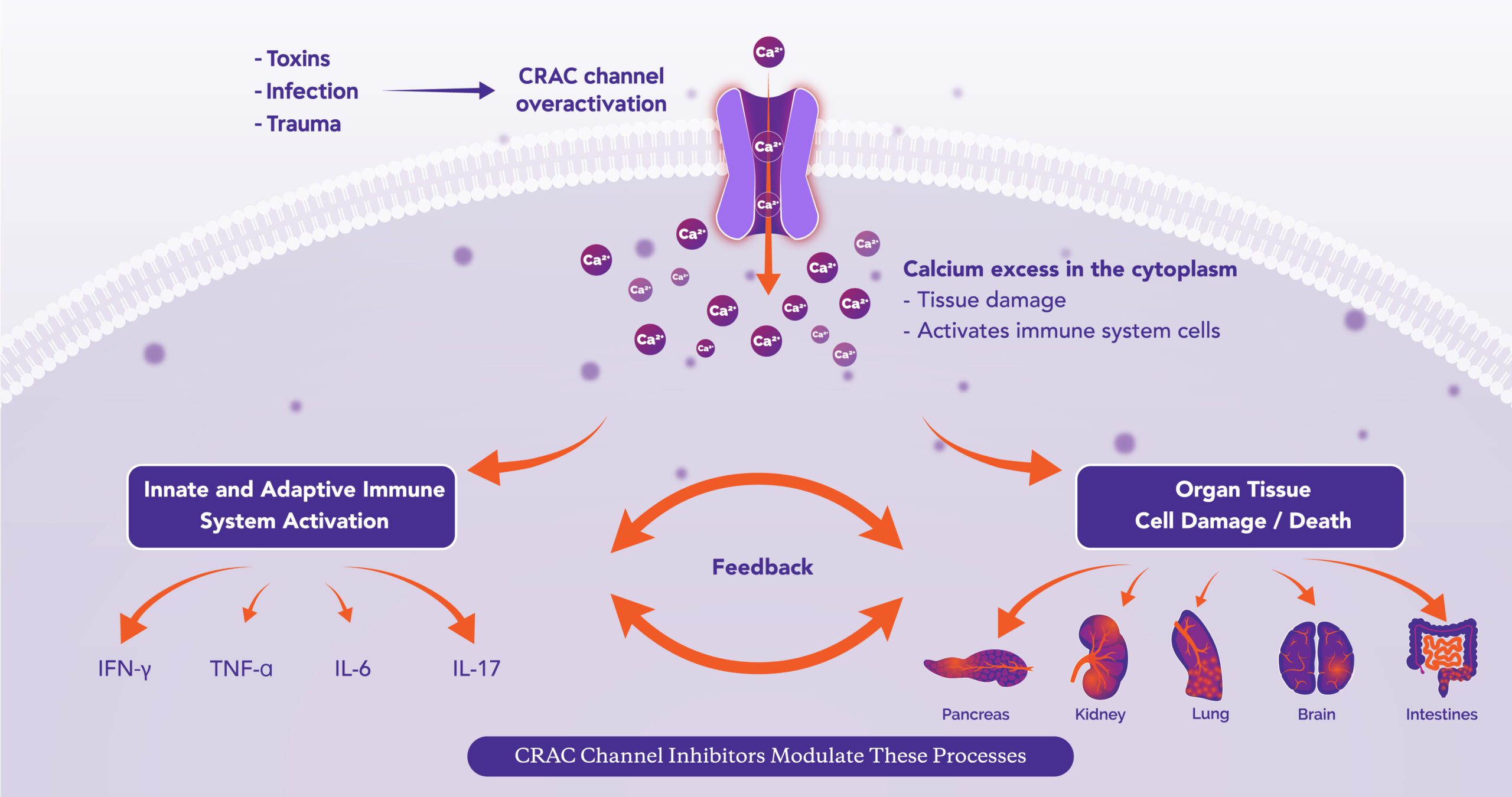
The Leader in CRAC Channel Inhibition: We are developing a new class of therapies that are designed to modulate the immune response and protect against tissue cell injury, two processes central to acute critical care illnesses like AKI and AP.
Our lead compound, Auxora, has the potential to tap into
the broad ICU market and improve outcomes meaningfully in
life-threatening conditions for which there are currently
no approved disease-modifying treatments.
AP hospitalizations per year in the US, with 170K having predicted severe disease
- Standard of care: Supportive care – IV fluids, pain medication, and nutrition
- Severe complications include pancreatic necrosis, life-threatening distal organ failure, and acute respiratory distress syndrome (ARDS)
- A major economic burden: >1M+ patient days in hospital per year and >$3B cost per year
Stage 2 or 3 AKI patients in the ICU each year, 800K of which have concurrent respiratory failure
- Standard of Care: Supportive care, treatment of complications, and dialysis
- Disease can progress to:
- Chronic kidney disease
- End stage renal disease
- Eventual Death
- 90-Day Mortality: ~50%

Auxora™
Auxora™, an intravenous formulation of the proprietary small
molecule calcium release-activated calcium (CRAC) channel
inhibitor zegocractin, is in development for the treatment
of AKI with Respiratory Failure and AP.


A Leadership Team
Dedicated to Science and
Innovation.
The CalciMedica team is comprised of scientists, physicians,
drug development experts and entrepreneurs dedicated to
the development of CRAC channel inhibitors for acute
inflammatory and immunologic diseases.


“Our focus at CalciMedica is on developing and bringing to market an entirely new class of therapies – CRAC channel inhibitors – to fight life-threatening acute inflammatory diseases, including AKI and AP. We are dedicated to addressing the high unmet need for safe and effective therapies to benefit patients with these acute illnesses for which there are currently no approved treatments.”

