
Acute Pancreatitis
Acute Pancreatitis represents an unmet need in critical care medicine as there are no approved disease-modifying therapies.
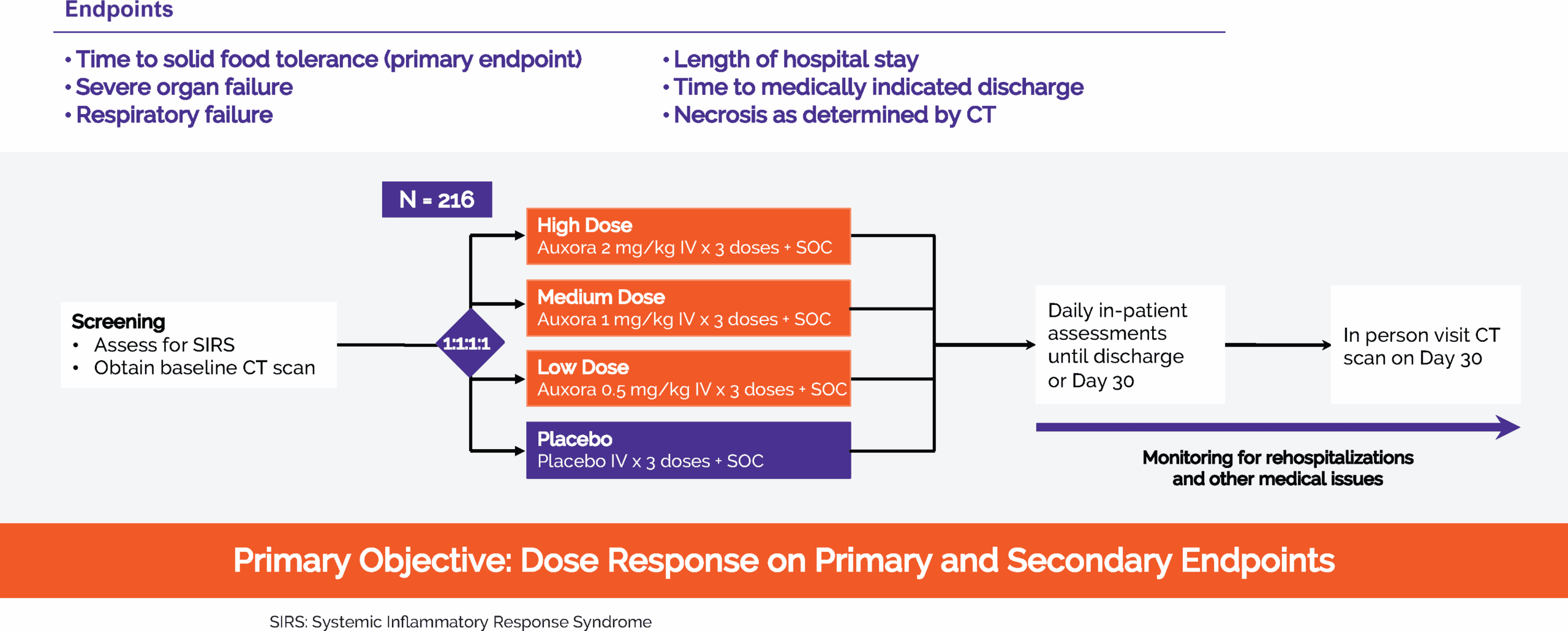
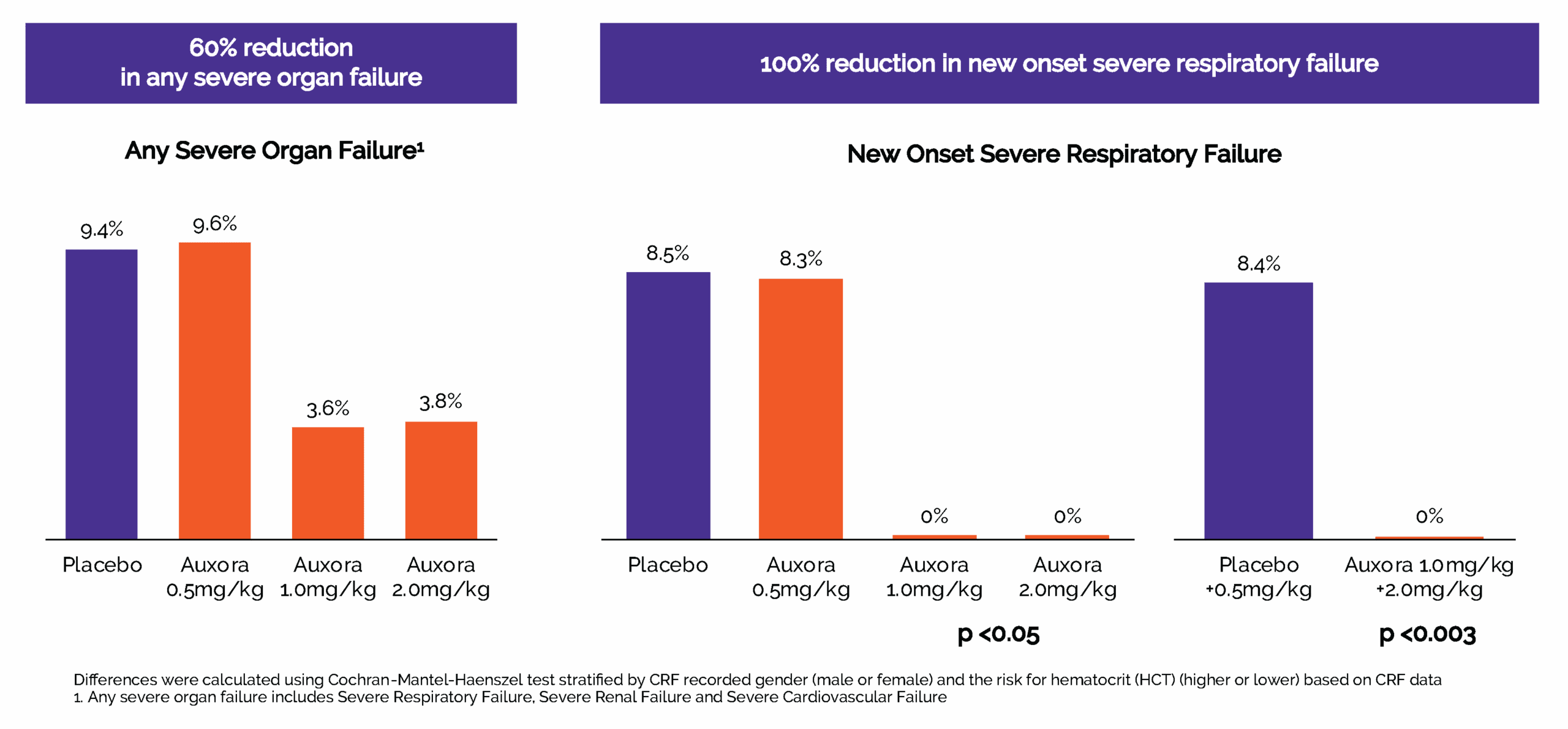
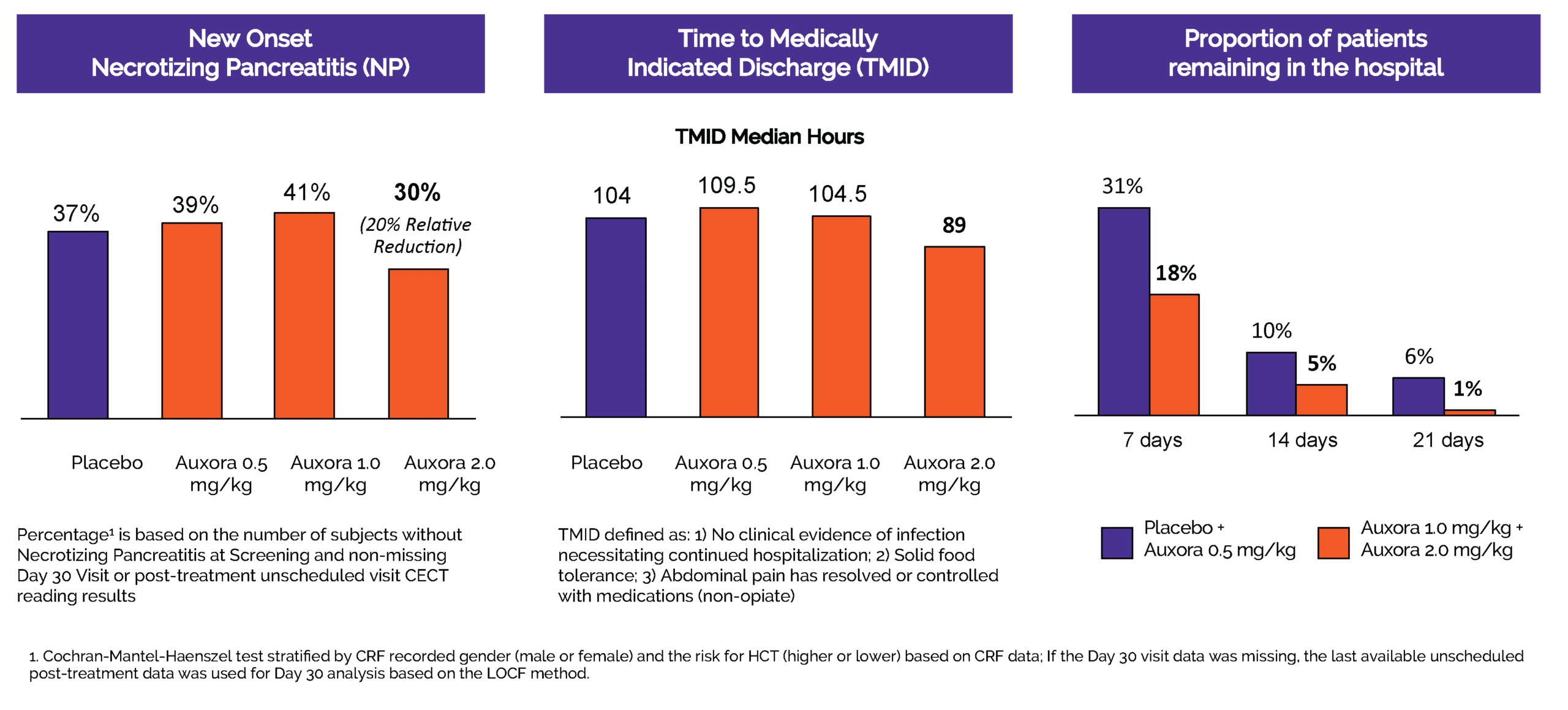
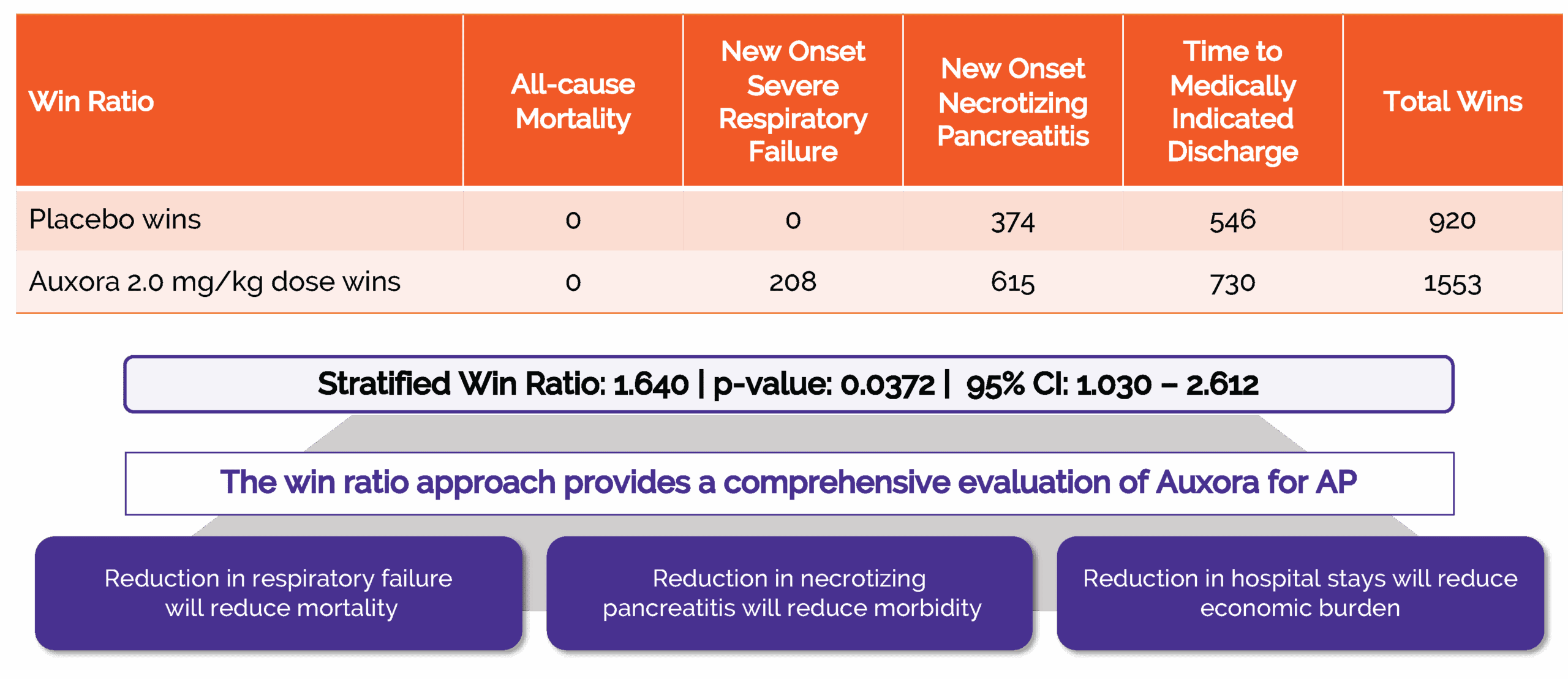
In 2024, we completed CARPO, an international, randomized, double-blind placebo-controlled Phase 2b trial evaluating AuxoraTM versus placebo in 216 patients with AP and accompanying SIRS. The trial met its primary objective with a statistically significant dose response in median time to solid food tolerance in a prespecified subgroup of patients with hyper-inflamed AP. Additionally, the medium and high doses of Auxora both showed a reduction in severe organ failure and, specifically, a 100% relative risk reduction compared to placebo in new-onset severe respiratory failure. This effect was statistically significant for the high and medium dose arms individually (p<0.05) and for the combined high and medium doses of Auxora patients compared with the combined placebo and low dose of Auxora patients (p=0.0027). There was an approximately 20% relative risk reduction for new onset necrotizing pancreatitis for patients in the high dose group compared to placebo patients and these high dose patients also had median time to medically indicated discharge of 15 hours less than placebo patients. Finally, a stratified win-ratio analysis of certain key endpoints gave a stratified 1.640 win-ratio benefit (p=0.0372) for the high dose patients compared to placebo patients.
We are in discussions with the FDA regarding the design of a pivotal program in AP.
Reduction in new onset respiratory failure
Reduction in severe organ failure
Reduction in necrotizing pancreatitis
Reduction in hospital stays > 21 days


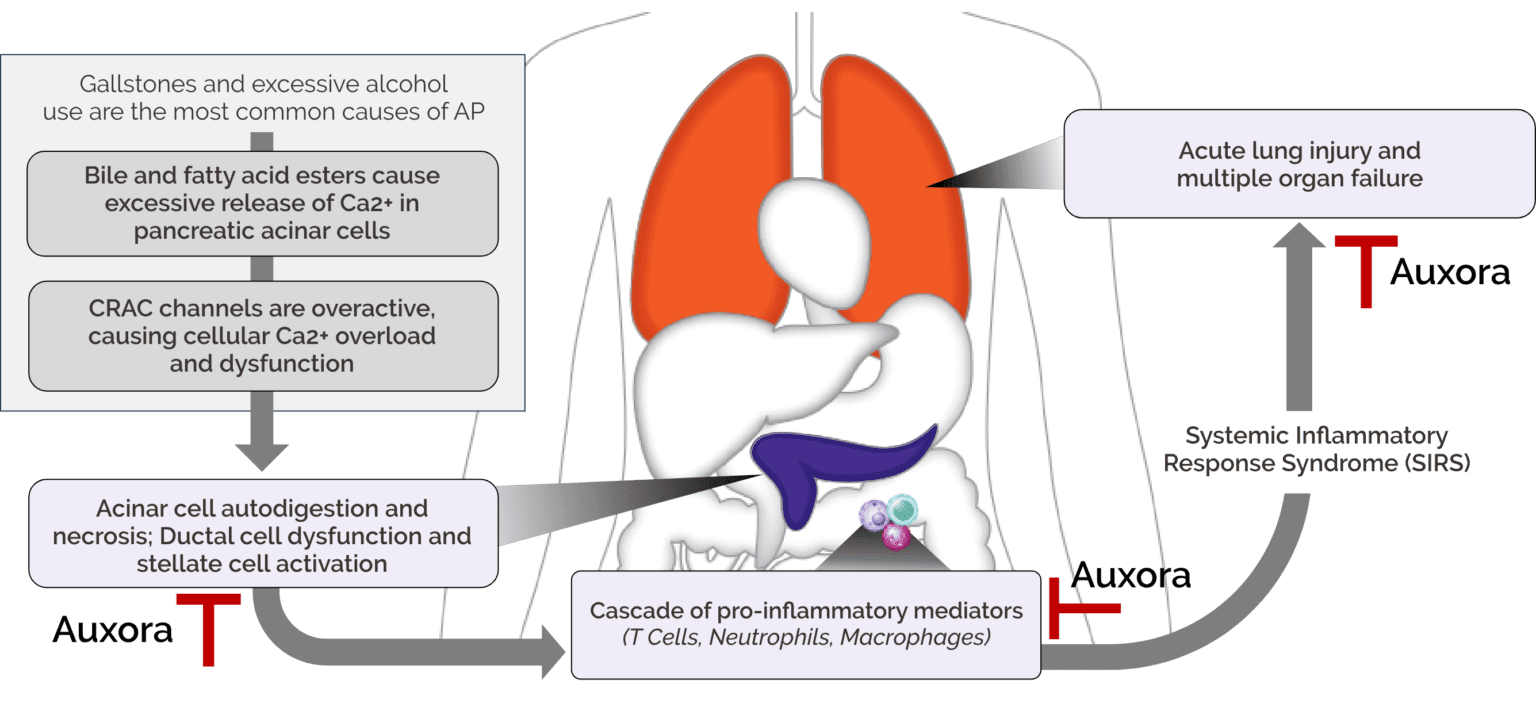
Acute pancreatitis (AP) is an acute inflammatory process in the pancreas that presents as severe upper abdominal pain, often accompanied by nausea and vomiting. During episodes of the disease, the inflammation of the pancreas can lead to pancreatic cell death and tissue necrosis as well as systemic inflammation. Normal pancreatic functions, such as the secretion of digestive enzymes into the gut that are required to break down proteins, carbohydrates and fats, are compromised.


The challenge for AP is that severe systemic complications can arise because of the acute inflammatory response to inflammation that occurs in the pancreas. Systemic Inflammatory Response Syndrome, or SIRS, driven by pancreatic tissue damage can amplify the immune response and lead to failure in organs beyond the pancreas, including the lung, kidney and heart. AP patients who have certain risk factors like SIRS and significant pancreatic inflammation at diagnosis are more likely to develop life-threatening severe AP than patients without SIRS.
There are no approved therapeutics for AP.
Hospitalizations per year for AP
~170K hospitalizations per year for AP with SIRS
~60K develop severe disease
>1M+ patient days in hospital due to a range of complications, including organ failure, necrosis of the pancreas, and mortality
Auxora in Acute Pancreatitis
Our Clinical Studies in AP
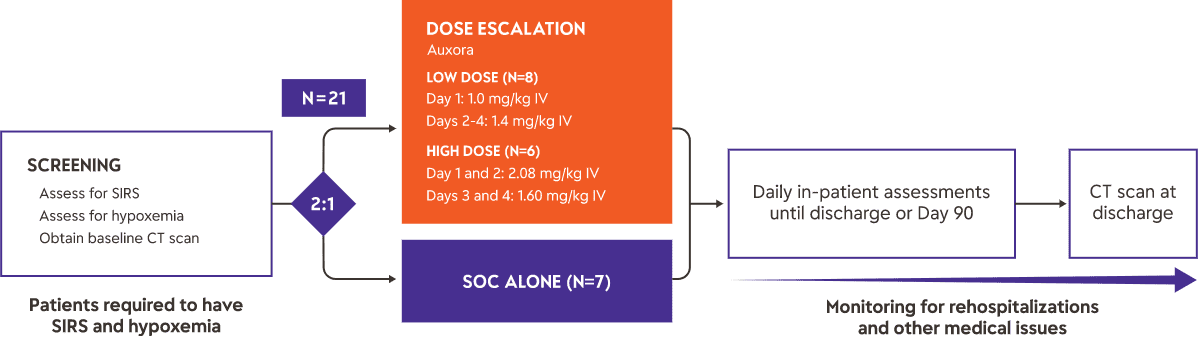
Acute Pancreatitis Phase 2a Clinical Trial
Study Design
We completed a Phase 2a clinical trial of Auxora™ in 21 patients with AP with predicted severe disease as determined by the presence of SIRS and hypoxemia. Fourteen patients received Auxora™ plus standard of care, typically aggressive fluid resuscitation and pain medication, at two different dose levels, and seven received standard of care only. The Auxora™ treated patients received a daily IV dose of Auxora™ for up to four days. As an early-stage proof of concept trial, this trial was not powered for statistical significance for any of the endpoints.
Results
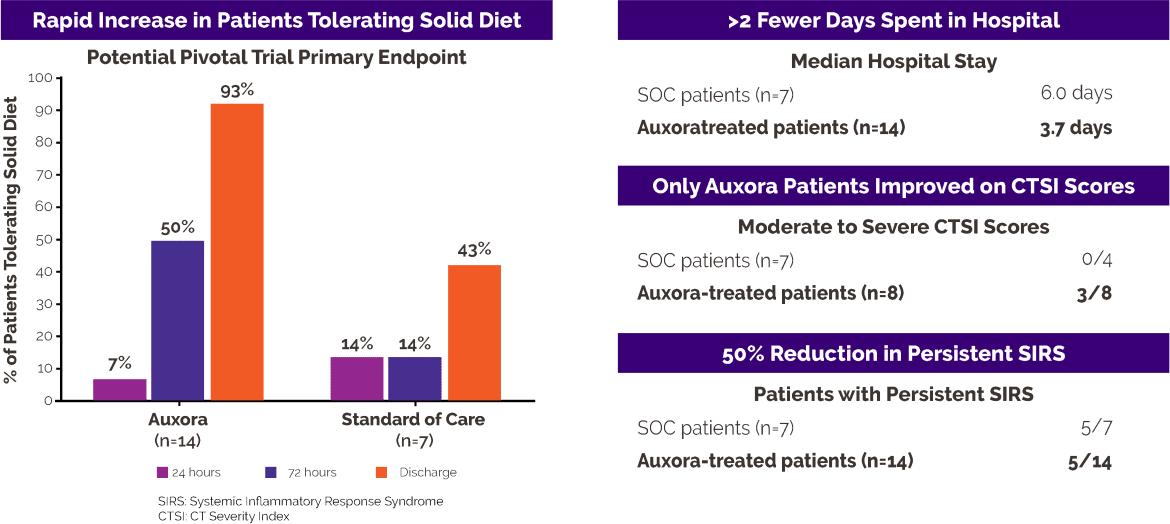
The primary symptom associated with AP is severe upper abdominal pain. Patients with AP are unable to tolerate any solid food without an increase in pain or the occurrence of nausea or vomiting until the pancreatitis resolves. In this clinical trial, only one patient in the Auxora™ treated group (7%) and one patient in the SOC group (14%) were tolerating solid food at study entry. After 72 hours, seven of 14 (50%) Auxora™ patients were tolerating solid food while only one of seven standard of care patients was tolerating solid food. At the time of hospital discharge, 13 of 14 (93%) Auxora™ patients could tolerate solid food compared to three of seven (43%) SOC patients.
Patients treated with Auxora™ were discharged from the hospital after a median of 3.7 days compared to SOC patients, who had a median stay of 6.0 days.
Persistent SIRS, defined as SIRS lasting more than 48 hours, is an indication of continued activation of inflammatory pathways in patients with AP, and SIRS is predictive for the development of organ failure. All patients in this AP trial had SIRS at enrollment. Only five of 14 patients (36%) treated with Auxora™ developed persistent SIRS, while five of the seven patients (71%) treated with SOC alone had persistent SIRS.
For more details on the clinical trial, please visit: A Study of Auxora in Patients With Acute Pancreatitis and Accompanying SIRS.
Information on our Expanded Access Policy can be obtained through the following link.
CARPO Phase 2b clinical trial in patients with AP and accompanying SIRS
Study Design
The target population for Auxora™ in the recently completed Phase 2b CARPO Clinical trial is AP patients with accompanying SIRS, which we estimate to be approximately 170,000 patients per year in the United States.
For more details on the clinical trial, please visit: A Study of Auxora in Patients with Acute Pancreatitis and Accompanying SIRS (CARPO).
Information on our Expanded Access Policy can be obtained through the following link.
CARPO Phase 2b clinical trial in AP with SIRS
Results
Auxora High and Medium-Doses reduced severe organ failure and completely prevented new onset severe respiratory failure
Auxora High-Dose showed improvements in key secondary endpoints
Integration of key endpoints into Win Ratio demonstrates potential benefits of Auxora High-Dose compared to Placebo


Oral CRAC channel inhibitors.
Solving The Unmet Need For
Patients With Chronic
Inflammatory Disease, such as
Chronic Pancreatitis
Beyond Auxora, we are also developing CRAC channel inhibitors for use in chronic inflammatory indications such as chronic pancreatitis that would be administered as oral agents. We are currently conducting IND-enabling preclinical testing on several CRAC channel inhibitors for use in chronic inflammatory diseases.
Beyond Auxora, we are also developing CRAC channel inhibitors for use in chronic inflammatory indications such as chronic pancreatitis that would be administered as oral agents. We are currently conducting IND-enabling preclinical testing on several CRAC channel inhibitors for use in chronic inflammatory diseases.

